July 2008
Volume 24, Number 8
New York State
Medicaid Update
The official newsletter of the New York Medicaid Program
David A. Paterson, Governor
State of New York
Richard F. Daines, M.D. Commissioner
New York State Department of Health
Deborah Bachrach, Deputy Commissioner
Office of Health Insurance Programs
Policy and Billing Guidance
Family Health Plus Pharmacy Benefit
Effective October 1, 2008, the pharmacy benefit for Family Health Plus managed care enrollees will be "carved-out" of the managed care plan benefit package.
Payment Edits Policy for Treatment of an Emergency Medical Condition
Review this important policy regarding Treatment of an Emergency Medical Condition.
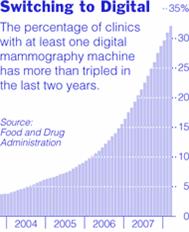
NYS Medicaid Coverage Policy for Digital Mammography
Review this important policy regarding digital mammography and what Medicaid covers.
Provider Bulletins
Policy updates for all providers.
Hearing Aid Devices Soon To Be Approved Through DVS
On September 1, 2008 hearing aids for children and adults will be approved through DVS.
Pharmacy News
New Drugs Added To Clinical Drug Review Program
Effective July 30, 2008 new drugs will be added to the Clinical Drug Review Program (CDRP).
EPIC Update - New York's Prescription Coverage for Seniors
On October 1, 2008 EPIC will require the substitution of brand name multi-source drugs with a generic equivalent.
Pharmacy Co-Payment Changes
On July 1, 2008 co-payments for "preferred" brand name prescription drugs will be reduced from $3 to $1.
Reimbursement Changes for Prescription Drugs...
Recently enacted legislation has resulted in changes to the Medicaid pharmacy reimbursement rates.
Unclassified Drug Code Now Available for Billing
Effective for dates of service on and after July 1, 2008, unclassified drugs will be available for billing.
General Information
Don't Be Silent About Smoking
Talk to your patients about the dangers of smoking.
Policy & Billing Guidance
Family Health Plus Pharmacy Benefit
Return to Table of Contents
Effective October 1, 2008, the pharmacy benefit for Family Health Plus managed care enrollees will be "carved-out" of the managed care plan benefit package and will be administered by the Medicaid fee-for-service program. Family Health Plus enrollees will be issued a Medicaid Benefit Identification Card to obtain their pharmacy benefit.

If enrollees are already using a Medicaid Benefit Identification Card to obtain other benefits, they can use the same Benefit Identification Card to access their pharmacy benefits on or after October 1, 2008. If they currently use a Family Health Plus managed care identification card to access their pharmacy benefits, they must continue to do so until October 1, 2008.
The pharmacy benefit includes:
- Prescription drugs
- Insulin and diabetic supplies currently covered as a pharmacy benefit by Medicaid (e.g., insulin syringes, blood glucose test strips, lancets, alcohol swabs)
- Smoking cessation agents, including OTC products
- Select over-the-counter medications covered on the Medicaid Preferred Drug List (Prilosec OTC, loratadine, Zyrtec)
- Hearing aid batteries
- Enteral formulae with prior authorization (1-866-211-1736)
- Drug co-payments for Family Health Plus enrollees will not change. Co-payments will remain at $6.00 for brand-name drugs, $3.00 for generic drugs, $1.00 for diabetic supplies, hearing aid batteries and enteral formulae, and $0.50 for covered over-the-counter drugs. For more information about Family Health Plus co-payments, please refer to the April 2006 Medicaid Update, "Family Health Plus Co-payment Information."
- Prescriptions for Family Health Plus enrollees will be subject to Medicaid's Preferred Drug Program, Clinical Drug Review Program, and the Mandatory Generic Drug Program. Prescriptions will also be subject to all Medicaid program requirements. Providers may need to request prior authorization for coverage of some drugs at 1-877-309-9493, https://newyork.fhsc.com or http://www.nyhealth.gov/health_care/medicaid/program/pharmacy.htm
- With the exception of controlled substances, prescriptions must be filled within 60 days and are valid for 6 months from original prescription date with up to 5 refills.
Drugs administered in the physician's office (J-Code drugs) remain in the managed care benefit package and should continue to be billed to the enrollee's Family Health Plus managed care plan.
Questions? Contact Medicaid Pharmacy Policy & Operations staff at (518) 486-3209.
Policy & Billing Guidance
Payment Edits Policy for Treatment of an Emergency Medical Condition
Return to Table of Contents
Payment edits were implemented in November 2007 to correct errors in claiming for services provided to undocumented immigrants and/or temporary non-immigrants, which were not true medical emergencies as defined under federal and State law, but were billed with the emergency indicator set to "Yes".
What services do not meet the definition of an Emergency Medical Condition?
When an enrollee is eligible for Emergency Services Only, Medicaid no longer covers the costs for or the transportation to the following:
- rehabilitation services (including physical, occupational and speech therapies). These services do not fall under the definition of treatment of an emergency medical condition.
Current Medicaid policy states that certain types of care provided to chronically ill persons are beyond the intent of the federal and State laws which allow Medicaid to pay for the treatment of medical emergencies. Such care includes:
- alternate level of care in a hospital,
- nursing facility services, and
- home care (including but not limited to, personal care services, home health services and private duty nursing).
Care and services related to an organ transplant procedure are not included in the federal definition of treatment for an emergency medical condition.
What is the Certification of Treatment of an Emergency Medical Condition?
Temporary non-immigrants and undocumented immigrants applying for coverage for the treatment of an emergency medical condition must complete the appropriate Medicaid application and sign the LDSS-3955 (Upstate) or MAP-2151 (NYC): "Certification of Treatment of Emergency Medical Condition".
If the immigrant is unable to sign, his or her authorized representative may sign on the immigrant's behalf. Signing the form authorizes the local department of social services to request information regarding the emergency medical treatment. It also gives the physician or facility permission to provide such information.
The attending physician must in all cases, make the decision as to whether or not the medical treatment is for an emergency medical condition. The attending physician must complete the entire form and sign and date the certification and return it to the local department of social services.
The LDSS-3955/MAP-2151 form has space to accommodate up to four coverage periods "From-To-Date(s)" of Treatment/Hospital Stay) that may be entered by the provider. A new LDSS-3955 or MAP-2151 application must be submitted for subsequent or continuing treatment for an emergency medical condition.
Each person's "emergency" is unique and the coverage period under the definition of emergency medical condition is limited and date specific.
Therefore, Medicaid coverage for emergency care must be a specific period of time in the past (i.e., at least one day prior to the application date).
Medicaid payment for emergency services is limited to the day treatment was initiated and the following period of time in which the necessity for emergency services exists (e.g., the date of admission through the date of discharge from the hospital).
Please note:
The MA coverage dates to be entered on the LDSS-3955/MAP-2151 (From Date) begins with the first day of the emergency service. The maximum period of time for which "emergency treatment" (07 coverage) that may be entered from one submission of the LDSS-3955/ MAP-2151 form, is 90 days. This can be a combination of retroactive, current and prospective coverage. Future (prospective) coverage may not exceed 60 days.
How to indicate an emergency on a claim
The code used to indicate an emergency on a claim depends upon which method the provider has used to submit a bill. To receive a timely payment for Medicaid covered services, the following procedure
must be followed:
- A provider billing on the eMedNY 150001 paper claim form must check the "YES" box in field 16A - "Emergency Related"
- If the provider is billing electronically, a "Y" must be entered in the appropriate field of the SV109 segment of the 2400 Loop in the X12 837P format. Inpatient and outpatient hospital providers, including emergency room and freestanding clinics must by law bill electronically.
The new edit looks at the diagnosis and procedure codes to see if they are on file as emergency indicator "Yes" (Y). The emergency indicator by itself no longer allows a claim to be paid. When an enrollee has "07" coverage (Emergency Services Only), the emergency indicator must be entered on the claim and the diagnosis code, procedure code or NDC code must be flagged as an emergency by the Department of Health.
The current format is the HIPAA compliant 837I format. For these providers, the Type of Admission code identifies if the service was related to an emergency. The current emergency type of admission code is '1'. If the immigrant is denied Medicaid coverage, payment should be obtained from the patient.
Additional information regarding proper completion of claims is available online at: http://www.emedny.org/hipaa/emedny_transactions/transactions.html
Questions related to the billing process for these services should be addressed to the eMedNY Call Center at: (800) 343-9000
Questions regarding immigrant eligibility for Medicaid may be directed to the Bureau of Medicaid and Family Health Plus Enrollment at: (518) 474-8887

Would you like to receive the Medicaid Update newsletter up to three weeks sooner?
Sign up for electronic distribution today!
Simply send your e-mail address to: medicaidupdate@health.state.ny.us.
Policy & Billing Guidance
NYS Medicaid Coverage Policy for Digital Mammography
Return to Table of Contents
Mammography is the best available tool for early detection of breast cancer, when it is more effectively treated.1 One drawback of standard film mammography is reduced sensitivity in women with radiographically dense breasts. Digital mammography allows the degree of contrast in the image to be manipulated so that contrast can be increased in the dense areas of the breast. The Digital Mammographic Imaging Screening Trial (DMIST) demonstrated that digital mammography was superior to film mammography for breast cancer screening in a subset of asymptomatic women. 2
- Women who are premenopausal or perimenopausal
- Women who are under age 50
- Women who have radiographically dense breast tissue
Final results from the Oslo II study in Norway showed that full-field digital mammography with independent double-reading yielded a significantly higher cancer detection rate than film mammography in a population-based screening program, whereas positive predictive values were comparable for the two imaging modalities. 3
In the diagnostic setting, no data are available to suggest that any specific subgroup(s) may benefit from digital mammography rather than standard film mammography.
NYS Medicaid Coverage
(effective for dates of service on or after 7/1/08)
Screening Mammography
NYS Medicaid covers screening mammography by either film (CPT code 77057) or digital (HCPCS code G0202).
Diagnostic Mammography
NYS Medicaid covers diagnostic mammography by either film (CPT codes 77055 [unilateral], 77056 [bilateral]) or digital (HCPCS codes G0206 [unilateral], G0204 bilateral]).
Digital Mammography
Medicaid Coding Rules (effective for dates of service on or after 7/1/08)
1. Enhanced reimbursement is available for screening digital mammography, when medically indicated. Screening digital mammography should be reported with:
- G0202 Screening mammography, producing direct digital image, bilateral, all views
Standard film screening mammography should be reported with: - 77057 Screening mammography; bilateral
2. Enhanced reimbursements are not available for the following diagnostic digital mammography codes. These codes will be priced at the same rate as 77055 and 77056, respectively. Diagnostic digital mammography should be reported with one of the following codes:
- G0206 Diagnostic mammography, producing direct digital image, unilateral, all views
- G0204 Diagnostic mammography, producing direct digital image, bilateral, all views
Standard film diagnostic mammography should be reported with one of the following codes: - 77055 Mammography; unilateral
- 77056 Mammography; bilateral
3. When one of the above HCPCS codes for digital mammography (G0202, G0204, G0206) is reported, it is not permissible to simultaneously report any of the following CPT codes:
- 77055 Mammography; unilateral
- 77056 Mammography; bilateral
- 77057 Screening mammography; bilateral
4. Additional reimbursement for computer-assisted detection is available for the following add-on codes in conjunction with film mammography codes as indicated below.
- 77051 computer-aided detection (computer algorithm analysis of digital image data for lesion detection) with further physician review for interpretation, with or without digitalization of film radiographic images; diagnostic mammography (use 77051 in conjunction with 77055, 77056)
- 77052 computer-aided detection (computer algorithm analysis of digital image data for lesion detection) with further physician review for interpretation, with or without digitalization of film radiographic images; screening mammography (use 77052 in conjunction with 77057)
References
(1) Smith RA, Cokkinides V, Eyre HJ. American Cancer Society Guidelines for the Early Detection of Cancer, 2006. CA Cancer J Clin 2006;56:11-25.
(2) Pisano ED, Gatsonis C, Hendrick E, Yaffe M, Baum JK, Acharyya S, Conant EF, Fajardo LL, Bassett L, D'Orsi C, Jong R, Rebner M. Diagnostic Performance of Digital versus Film Mammography for Breast-Cancer Screening. NEJM 2005; 353;1773-1783
(3) Skaane P, Hofvind S, Skjennald A. Randomized Trial of Screen-Film versus Full-Field Digital Mammography with Soft Copy Reading in Population-Based Screening Program: Follow-up and Final Results of Oslo II Study. Radiology 2007; 244:708-717.
Questions? Contact the Division of Financial Planning and Policy at (518) 473-2160.
Policy & Billing Guidance
Effective for dates of service on and after September 1, 2008, hearing aids for children and adults will be approved through the automated electronic Dispensing Validation System (DVS). A DVS request is submitted in "real time", which means that the status of the request will be available by the end of your DVD session. The eMedNY claims system will no longer bypass prior approval when an order for a hearing aid originates from a Physically Handicapped Children's Program Approved Speech and Hearing Center.
Important information on this change:
- Centers, hearing aid vendors and dispensing audiologists will need to obtain an electronic DVS number prior to providing a hearing aid and must report it on the claim for payment to be made.
- DVS checks service limits on the procedure code requested, including frequency, units and age. If service limits are not exceeded, an immediate authorization number is returned. If service limits are exceeded, a prior approval must be requested (see chart below).
- A hearing aid DVS authorization will be granted for an approved period of service of 365 days, and can be cancelled by the provider within 90 days of the authorization date.
- For monaural aids, the left (LT) and the right (RT) modifiers must be included in the DVS and reported on the claim. A separate DVS is required for LT) and (RT) modifiers.
- On the ordering provider identification field on prior authorizations and claims, the actual provider ID number of the qualifying ordering provider must be entered. Licensed audiologists, physicians and nurse practitioners are qualified to order hearing aids.
- No facility or center provider identification numbers will be allowed in the ordering provider field due to HIPAA/NPI requirements.
- The ordering and dispensing provider ID number must be reported on the DVS request and the claim.
- To obtain a National Provider ID, providers must visit the federal government's Web site at https://nppes.cms.hhs.gov or call 1-800-465-3203 or 1-800-692-2326 for hearing impaired.
- NYS Medicaid enrolled providers must report their NPI to NYS Medicaid via the Web site http://www.emedny.org. Audiologists who are not enrolled as Medicaid providers do not have to report NPI to NYS Medicaid but must still obtain an NPI from the federal NPPES Web site above.
- All other requirements, coverage and policies relating to hearing aids remain in effect. See the Hearing Services Provider Manual at http://www.emedny.org/providerManuals/HearingAid/index.html
- Please note the source of recommendations for hearing aids for Medicaid patients under 21 years of age on page 7 of the Hearing Aid Policy Guidelines. Recommendations for these patients may come from a PHCP approved speech and hearing aid center or other approximately licensed or credentialed provider.
The following chart illustrates the service limits used by DVS when a hearing aid is requested. If the service limits are exceeded, prior approval is required. When a hearing aid reaches its life expectancy, but is in good working order and meets the beneficiary's medical needs, it should not automatically be replaced.
| Code | Description | Service Limits | ||
|---|---|---|---|---|
| Frequency | Age | Units | ||
| #V5030 | Hearing aid, monaural, body worn, air conduction | 1/5yrs | any | 1 |
| #V5040 | Hearing aid, monaural, body worn, bone conduction | 1/5yrs | any | 1 |
| #V5050 | Hearing aid, monaural, in the ear | 1/5yrs | any | 1 |
| #V5060 | Hearing aid, monaural, behind the ear | 1/5yrs | any | 1 |
| #V5070 | Glasses, air conduction | 1/5yrs | any | 1 |
| #V5080 | Glasses, bone conduction | 1/5yrs | any | 1 |
| #V5120 | Binaural, body | 1/5yrs | 0-20 | 1 |
| #V5130 | Binaural, in the ear | 1/5yrs | 0-20 | 1 |
| #V5140 | Binaural, behind the ear | 1/5yrs | 0-20 | 1 |
| #V5150 | Binaural, glasses | 1/5yrs | 0-20 | 1 |
| #V5170 | Hearing aid, CROS, in the ear | 1/5yrs | any | 1 |
| #V5180 | Hearing aid, CROS, behind the ear | 1/5yrs | any | 1 |
| #V5190 | Hearing aid, CROS, glasses | 1/5yrs | any | 1 |
| #V5210 | Hearing aid, BICROS, in the ear | 1/5yrs | 0-20 | 1 |
| #V5220 | Hearing aid, BICROS, behind the ear | 1/5yrs | 0-20 | 1 |
| #V5230 | Hearing aid, BICROS, glasses | 1/5yrs | 0-20 | 1 |
| #V5246 | Hearing aid, digitally programmable analog, monaural, ITE (in the ear) | 1/5yrs | any | 1 |
| #V5247 | Hearing aid, digitally programmable analog, monaural, BTE (behind the ear) | 1/5yrs | any | 1 |
| #V5252 | Hearing aid, digitally programmable, binaural, ITE | 1/5yrs | 0-20 | 1 |
| #V5253 | Hearing aid, digitally programmable, binaural, BTE | 1/5yrs | 0-20 | 1 |
| #V5256 | Hearing aid, digital, monaural, ITE | 1/5yrs | any | 1 |
| #V5257 | Hearing aid, digital, monaural, BTE | 1/5yrs | any | 1 |
| #V5260 | Hearing aid, digital, binaural, ITE | 1/5yrs | 0-20 | 1 |
| #V5261 | Hearing aid, digital, binaural, BTE | 1/5yrs | 0-20 | 1 |
#=DVS required
Providers may use one of two methods to obtain a DVS prior authorization number:
- Medicaid Eligibility Verification System (MEVS) card swipe terminal, the VeriFone Omni (instructions for use located at: http://www.emedny.org/ProviderManuals/AllProviders/Omni_3750_Supplemental_Guide/1_3/Omni3750_Supplemental_Guide.html
- e-PACES free personal computer software (instructions located at: http://www.emedny.org/HIPAA/SupportDocs/ePACES.html
- Providers not prepared to access DVS on or after Sept. 1, 2008 may submit a DVS override through the prior approval process during a six month transition period. Providers are strongly encouraged to access DVS as soon as possible to expedite the provision of medically necessary services to beneficiaries.
- Providers receiving a service limit rejection through DVS may submit a DVS override at any time through the prior approval process with documentation attached as to why the service limits need to be overridden.
- Instructions on prior approval submission are available at: http://www.emedny.org/ProviderManuals/HearingAid/PDFS/HearingAid_PA_Guidelines.pdf
- For more information and assistance with obtaining a DVS through e-PACES and the Omni terminal or to obtain Prior Approval Request Form eMedNY 283201 (paper form) for DVS overrides, contact Computer Sciences Corporation (CSC) at (800) 343-9000.
- For information regarding general Medicaid coverage of hearing aids or a specific prior approval submission, call the Medical Prior Approval Bureau at (800) 342-3005, Option #1.
Provider Bulletins
Important Reminder
Hospital, Laboratory, & Ambulatory Care Providers
Return to Table of ContentsAncillary services that are already included in a facility's all inclusive DRG payment, such as laboratory tests, should not be billed on a fee-for-service basis. Services rendered to hospital inpatients should not be billed on an ordered ambulatory basis. This situation is considered to be a duplicate payment and therefore subject to recoupment.
* Please Note: Only certain HIV drugs may be billed in addition to the all-inclusive DRG, these are AZT, Pentamidine, and Ganciclovir.
For additional information regarding this article contact the Bureau of Policy Development and Coverage, at (518) 473-2160.
Billing Providers
*Please Note: An editing change is planned at eMedNY that may require a change in the way providers bill for services.
Effective July 17, 2008, claims on which the servicing provider ID the same as the billing provider ID will be denied. The provider's remittance statement will identify these denied claims as Edit 1357 denials (Provider ID and Service Provider ID identical). Only claims containing a provider identifier that is different than the billing provider's ID will be paid. If you are a solo practitioner billing for your own services, leave the servicing provider field blank.
*Reminder: When completing the servicing provider identification on a claim, it is important to use the correct MMIS ID, or license number and profession code, of the actual individual servicing the patient.
Questions? Please contact CSC Provider Relations at (800) 343-9000.
Pharmacy News

Effective July 30, 2008, the following drugs will be added to the Clinical Drug Review Program (CDRP).
- *exenatide (Byetta®)
- *fentanyl buccal tablet (Fentora®)
- *fentanyl lozenge (Actiq®)
- *lidocaine patch (Lidoderm®)
Prescriptions written for these drugs, on or after July 30, 2008, will require prior authorization. Prescribers and pharmacists will be receiving additional information by direct mail.
To view the most current listing of CDRP drugs, please visit: https://newyork.fhsc.com/providers/CDRP_about.asp
To access the most current CDRP instructions and worksheets, please visit: https://newyork.fhsc.com/providers/CDRP_forms.asp
To obtain prior authorization for a CDRP drug, please contact the clinical call center at (877) 309-9493 and follow the appropriate prompts. The clinical call center is available 24 hours per day, 7 days per week. Requests for prior authorization of CDRP drugs can not be faxed.
For certain drugs subject to the CDRP, only prescribers, not their authorized agents, can initiate the prior authorization process.
For clinical concerns or Clinical Drug Review Program questions, please call (877) 309-9493.
For billing questions, please call (800) 343-9000.
For Medicaid pharmacy policy and operation questions, please call (518) 486-3209.
UPDATE FROM EPIC - NEW YORK'S PRESCRIPTION COVERAGE FOR SENIORS
Return to Table of Contents
Mandatory Generic Program - Effective October 1, 2008, EPIC will require the substitution of brand name multi-source drugs with the generic equivalent on prescriptions covered only by EPIC. A brand name drug will only be covered if the prescriber obtains a prior authorization from EPIC. More details will follow in future issues of the Medicaid Update.
Coordination with Medicare Part D - Most EPIC members are enrolled in Medicare Part D, and use EPIC to help pay for their Part D coverage and out-of-pocket costs. When a Medicare Part D plan denies coverage of a drug before billing EPIC, pharmacies are expected to consult with the prescriber about a possible substitute covered by the primary plan. If an acceptable alternative is not available, or if the prescriber could not be reached before the senior needs their medication, the pharmacy should document the consultation (or attempt) in their records and bill EPIC for the drug. EPIC will then pursue an appeal with the Part D plan for a formulary exception.
Please be sure to refer any seniors struggling to pay out-of-pocket drug costs (deductibles, co-payments, non-covered drug costs or during the Medicare coverage gap) to EPIC. The EPIC Helpline can be reached by calling 1-800-332-3742.
Pharmacy News
Effective July 1, 2008, co-payments for "preferred" brand name prescription drugs will be reduced from three dollars ($3.00) to one dollar ($1.00). Co-payments for all other brand name, generic and over-the-counter drugs remain unchanged.
For services provided on and after July 1, 2008, the prescription drug co-payment structure is as follows:
| Prescription Item | Co-payment Amount | Co-payment Details |
|---|---|---|
| Brand-name prescription drugs | $3.00 | One co-payment charge for each new prescription and each refill. No co-payment for drugs to treat mental illness (psychotropic) and tuberculosis. See the list below for other conditions that do not require a co-payment. |
| "Preferred" brand-name prescription drugs | $1.00 | |
| Generic prescription drugs | $1.00 |
For a list of "preferred" brand-name prescription drugs or to obtain more information regarding the Preferred Drug Program go to https://newyork.fhsc.com or call (877) 309-9493.
In addition, the following continue to be exempt from co-payments:
- Beneficiaries younger than 21 years old.
- Beneficiaries who are pregnant. Pregnant women are exempt during pregnancy and for the two months after the month in which the pregnancy ends.
- Family planning drugs and supplies like birth control pills and condoms.
- Residents of an Adult Care Facility licensed by the New York State Department of Health (for pharmacy services only).
- Residents of a Nursing Home.
- Residents of an Intermediate Care Facility for the Developmentally Disabled (ICF/DD).
- Residents of an Office of Mental Health (OMH) or Office of Mental Retardation and Developmental Disabilities (OMRDD) certified Community Residence.
- Beneficiaries in a Comprehensive Medicaid Case Management (CMCM) or Service Coordination Program.
- Beneficiaries in an OMH or OMRDD Home and Community Based Services (HCBS) Waiver Program.
- Beneficiaries in a DOH HCBS Waiver Program for Persons with Traumatic Brain Injury (TBI).
It is anticipated that the co-payment adjustment to the claims system will be made before July 25, 2008. Prescriptions for brand-name drugs with dates of service on or after July 1, 2008 will automatically be reprocessed by Computer Sciences Corporation. Reprocessed claims will result in pharmacies being reimbursed for the $2.00 difference in the co-payment amount not included in the original claim transaction.
Pharmacies do not need to resubmit claims in order to receive the adjustment. The eMedNY point-of-service (POS) claims system will temporarily continue to deduct a co-payment amount of $3.00 for "preferred" brand name prescription drugs after the July 1, 2008 effective date. Pharmacies are asked to only collect $1.00 from the patient for "preferred" brand name prescription drugs until the adjustment is made to the eMedNY POS claims system.
For Medicaid pharmacy policy questions, call (518) 486-3209 or e-mail ppno@health.state.ny.us
For Medicaid co-payment questions, call the Medicaid Helpline at (800) 541-2831.
Pharmacy News
Physicians, Nurse Practitioners, Podiatrists, and Article 28 Ordered Ambulatory Providers
Unclassified Drug Code Now Available for Billing
Return to Table of Contents
Effective for dates of service on and after July 1, 2008, J3490 (unclassified drugs) will be available for billing. The previous code used to bill unlisted drugs, 90779, will be discontinued.
This change is consistent with the definitions of the codes in CPT and HCPCS. J3490 will require by report billing. Documentation of medical necessity, identification of dose administered and evidence of acquisition cost will be required with submission of a paper claim.
Questions? Please call the Pre-Payment Review Group at (518) 474-8161.
Reimbursement Changes for Prescription Drugs Effective July 1, 2008
Return to Table of Contents

Recently enacted legislation has resulted in changes to the Medicaid pharmacy reimbursement rates. These changes will apply to payments that are issued for services provided on and after July 1, 2008.
Pharmacy reimbursement for prescription drugs under the New York State Medicaid program is limited to the lower of the:
- Dispensing pharmacy's usual and customary (U&C) price charged to the general public,
- Estimated acquisition cost (EAC) as established by the NYS Department of Health,
- Federal upper limit (FUL) when established by the Centers for Medicare and Medicaid, or
- State maximum acquisition cost (SMAC) when established by the NYS Department of Health.
- For Sole or Multi-source Brand Name Drugs the EAC is defined as the average wholesale price (AWP) of the prescription product minus sixteen and one-quarter percent (16.25%). State and federal requirements for the dispensing of multi-source brand name drugs must be met.
- For Multi-source Generic Drugs the EAC definition is unaffected by changes to the recently enacted legislation.
- There will be no change to the current reimbursement rates for specialized HIV pharmacies which meet specific programmatic and operational criteria.
Questions may be directed to the Medicaid Pharmacy Policy and Operations staff at: (518) 486-3209.

Resources .................................................................................................................................................
| NYS SMOKERS' QUITLINE (866) NY-QUITS or (866-697-8487) | AMERICAN CANCER SOCIETY (800) 227-2345 |
AMERICAN LUNG ASSOCIATION (800) 586-4872 |
| CENTERS FOR DISEASE CONTROL AND PREVENTION (800) CDC-4636 or (800-232-4636) | NATIONAL CANCER INSTITUTE (800) 4-CANCER or (800-422-6237) | For more information on the New York State Medicaid Smoking Cessation policy, please call the Bureau of Pharmacy Policy and Operations at (518) 486-3209. |

PROVIDER SERVICES
Return to Table of Contents
Missing Issues?
The Medicaid Update, indexed by subject area, can be accessed online at:
http://www.nyhealth.gov/health_care/medicaid/program/update/main.htm
Hard copies can be obtained upon request by emailing: medicaidupdate@health.state.ny.us
Office of the Medicaid Inspector General: http://www.omig.state.ny.us (518) 473-3782
Questions about an Article?
Each article contains a contact number for further information, questions or comments.
Questions about billing and performing EMEVS transactions?
Please contact eMedNY Call Center at: (800) 343-9000.
Provider Training
To sign up for a provider seminar in your area, please enroll online at: http://www.emedny.org/training/index.aspx
For individual training requests, call (800) 343-9000 or email:emednyproviderrelations@csc.com
Enrollee Eligibility
Call the Touchtone Telephone Verification System at any of the numbers below:
(800) 997-1111 (800) 225-3040 (800) 394-1234.
Address Change?
Questions should be directed to CSC at: (800) 343-9000.
Fee-for-Service Providers
A change of address form is available at:
http://www.emedny.org/info/ProviderEnrollment/index.html
Rate-Based/Institutional Providers
A change of address form is available at:
http://www.emedny.org/info/ProviderEnrollment/index.html
Comments and Suggestions Regarding This Publication?
Please contact the editor, Kelli Kudlack, at:
medicaidupdate@health.state.ny.us
Medicaid Update is a monthly publication of the New York State Department of Health containing information regarding the care of those enrolled in the Medicaid Program.


