Clinical Guidelines for the Medical Management of Hepatitis C
D. Diagnosis
1. Testing for HCV Infection
The recommendations for testing are derived from published evidence and expert opinions.22,23 There are many options for HCV testing and, as newer diagnostic technologies are introduced, HCV testing should become more streamlined. Also, some providers will have limited choices for testing because of laboratory testing protocols and reimbursement issues. Providers are advised to consult with their laboratories for information regarding available tests and testing protocols.
Recommendations
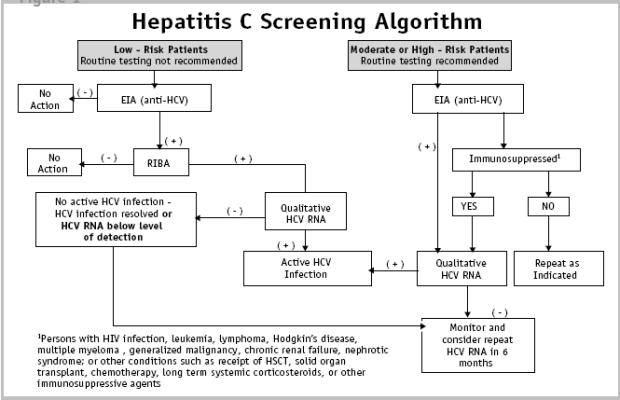
All patients suspected of having infection with HCV should be tested for antibody to HCV (anti-HCV) using an EIA (enzyme immunoassay) screening test. In low-risk patients with a positive EIA test, confirmatory testing with the recombinant immunoblot assay (RIBA) should be performed. For patients at low risk with a positive EIA and RIBA, confirmatory testing with a qualitative PCR test for detection of HCV RNA should be performed.
For patients at moderate or high risk and/ or unexplained elevated serum alanine aminotransferase (ALT ) value, a positive EIA should be followed by a qualitative test for HCV RNA in the blood.
For immunocompromised patients at high risk with unexplained elevated ALT value and a negative screening EIA, a qualitative test for detection of HCV RNA should be performed to diagnose HCV infection.
There is no recommendation for serial or periodic screening unless there has been repeat or ongoing high-risk behavior. Quantitative PCR HCV RNA tests should be obtained for patients who are candidates for antiviral therapy.
2. Testing for Chronic HCV Infection
The initial screening test to be used in all circumstances is a test for antibody to hepatitis C viral proteins (anti-HCV). These tests become positive as early as 8-10 weeks after infection, will be positive in 97% of patients by 6 months after infection, and probably will persist for life. Presence of anti-HCV does not define activity of infection. Up to 25% of patients will resolve infection spontaneously, but will still have detectable anti-HCV. Antibody tests currently recommended for anti-HCV screening include the EIA test and the more specific RIBA; the latter being used to confirm a positive EIA test in some situations (e.g., confirming EIA positivity in low-risk patients). These antibody tests are highly reliable for determining HCV infection at some time in the past.
Detection of HCV RNA in blood is the currently accepted "gold standard" for diagnosis of active HCV infection. Tests for HCV RNA are both qualitative and quantitative, vary in technical aspects, and report values differently.
3. Characteristics of Antibody Tests for the Diagnosis of Chronic HCV Infection
Currently available EIA tests are highly sensitive and useful for screening. However, among a population with low risk, even a highly sensitive test does not provide the desired predictive value of a positive test. The lower the likelihood of infection, the higher the risk of a false positive test. Conversely, the higher the risk of hepatitis C in the population, the greater the likelihood that a positive screening EIA test indicates infection. In populations with otherwise unexplained ALT elevation and at least one major risk factor for hepatitis C, the positive predictive value of a positive EIA is at least 95%. This value declines to less than 50% for patients with normal ALT levels and minimal or no risk factors.38
Two strategies have been employed to decrease the possibility of "false positive" EIA tests:
- Recalibration of the EIA test value called "positive" has allowed a higher degree of positive prediction for the test in general. This gives the EIA test excellent characteristics for use in appropriate populations, and simplifies the use of confirmatory tests.
- Confirmatory testing for more specific antibody to hepatitis C virus. The recommended test is the RIBA to confirm a positive EIA test in a patient with low risk for hepatitis C.
A relatively small fraction of the population perceived to be at risk might have a false negative screening test. These populations include patients with advanced HIV, hemodialysis patients and patients with profound immunosuppression due to solid organ transplantation.39 Also included, are patients infected and screened soon after exposure when an antibody response has not been mounted to detectable levels. When other clinical or laboratory findings support a possible diagnosis of hepatitis C, qualitative testing for HCV RNA by PCR or transcription mediated assay (TMA) is appropriate. (see Table 2).
4. Testing for Acute Hepatitis C
Between 1-8 weeks after transmission of hepatitis C, HCV RNA becomes detectable by PCR testing. Although most patients will have some liver function test (LFT) abnormalities from 6-12 weeks after transmission, only about a quarter will have the syndrome of malaise, abdominal pain, and jaundice that characterizes acute hepatitis C disease. By 12 weeks after development of hepatitis C viremia, up to 25% of patients will spontaneously and permanently clear the virus.40 Spontaneous clearance appears to be much more common in those with the syndrome of acute hepatitis C than in those with asymptomatic viremia.41 Development of hepatitis C antibodies occurs as early as 8-10 weeks after transmission.42 Some immunosuppressed individuals may not develop hepatitis C antibodies despite the presence of viremia.43
| Qualitative | Method; Manufacturer | Dynamic Range (IU/mL)* |
|---|---|---|
| Amplicor HCV test 2.0 | PCR; Roche | ≥50 |
| COBAS Amplicor HCV test 2.0 | PCR; Roche | ≥50 |
| VERSANT HCV RNA Assay | TMA; Bayer | ≥5 |
| Quantitative | Method; Manufacturer | Dynamic Range (IU/mL)* |
| Versanttm HCV RNA 3.0 | bDNA; Bayer | 615 - 77.7 x106 |
| §COBAS Amplicor HCV Monitortm | qPCR; Roche | 600 - 7 x 105 |
| COBAS TaqMantm HCV | qPCR; Roche | 10 - 2 x 108 |
| Celera HCV QT ASR | qPCR; Abbott | 25 - 5 x 108 |
| *Results may vary at the lower limit of detection depending on the laboratory performing the test §Research Use Only - Not approved for patient use bDNA: branched chain DNA PCR: polymerase chain reaction qPCR: quantitative polymerase chain reaction TMA: transcription mediated assay ASR: analyte specific reagent The use of proprietary names does not constitute endorsement by the NYS DOH. |
||
5. Summary of Available Tests for HCV Screening
A comparison of currently available tests for HCV RNA is shown in Table 2 and a testing algorithm is shown in Figure 1.
- EIA is a reproducible and inexpensive test suitable for screening. This test identifies if there is antibody to HCV present. The currently available third generation tests have a high specificity and sensitivity of greater than 99%.22,23 The high sensitivity and specificity may obviate the need for a confirmatory immunoblot assay in the patient with clinical liver disease, especially in high-risk patients for HCV.
- RIBA determines if there is antibody to the HCV present. This test is very sensitive and reliable, and can be used as a confirmatory test of the EIA. A positive RIBA is not diagnostic of active HCV infection since up to 25% of patients will clear HCV spontaneously after acute infection yet remain anti-HCV positive. The diagnosis of active HCV infection requires additional testing.
- PCR testing is used to determine the presence of HCV RNA and is an indication of active infection. There are two types of PCR tests, commonly called viral load tests: qualitative and quantitative (see Table 2).
- Qualitative PCR or TMA testing determines if HCV is present or not. Traditionally, qualitative tests have been more sensitive with a lower limit of detection of 5 IU/mL. Qualitative test results are reported as positive or negative and are not reported numerically. A single negative qualitative test does not exclude viremia, since viral load can have transient declines. The negative test may reflect that the viral load was below assay detection at that particular point in time.
- Quantitative PCR determines the amount of virus present. The lower limit of detection for earlier versions of these tests has been around 600 IU/mL. More recent versions are more sensitive with a broader dynamic range from around 5-25 IU/mL to > 108 IU/mL, depending on the laboratory.
Figure 1
These newer technologies may help streamline the testing process when used for confirmation of anti-HCV, therapeutic monitoring and test of cure.
- Genotype testing is widely available and is useful in treatment planning and for determining length and possible responses to treatment. Genotype testing should be done as part of the patient's initial evaluation once CHC has been confirmed.
- Liver chemistries, though inexpensive, are an insensitive means of assessing disease activity. Elevations of ALT and aspartate aminotransferase (AST) may indicate the presence of liver disease, but do not determine the type of liver disease, what has caused it, or the degree of damage to the liver.
6. Liver Biopsy
Recommendations
In patients with genotype 1 or 4, pretreatment liver biopsy should be performed to assess the likelihood of a sustained virologic response(SVR).
In patients with genotype 2 or 3, it may not be necessary to perform a liver biopsy.
Liver biopsy remains the only definitive test for evaluation of fibrosis stage, and fibrosis stage is the most reliable means to assess prognosis and provide information for decisions about the need for initiation of therapy. Because up to 80% of patients with HCV genotype 2 or 3 disease respond favorably to antiviral therapy, a decision to treat is more straightforward, and a pretreatment liver biopsy might not be necessary for those with genotype 2 or 3 disease. If performed, liver biopsies should be evaluated by pathologists with training and experience in hepatic histology.
A liver biopsy was regarded as an important parameter in helping to guide management and treatment, particularly at a time when response to treatment was low. More recently, with the improvement of treatment effectiveness, the value of the liver biopsy has been questioned because of the potential risks of the procedure and the concern of sampling error. This has prompted some to challenge the need for biopsy and to suggest that the procedure may not be necessary prior to treatment. At the present time, the American Association for the Study of Liver Disease (AASLD) recommends that regardless of the level of ALT, a liver biopsy should be done when the results will influence whether treatment is recommended. A biopsy, however, is not mandatory in order to initiate therapy.22
7. Noninvasive Testing to Assess Liver Fibrosis
Recommendation
The use of non-invasive tests to assess liver fibrosis is not yet recommended.
Various non-invasive tests are being investigated for staging degree of liver fibrosis. These tests may be used in decisions regarding whether or not to initiate antiviral therapy and to monitor the effects of such therapy. 44 An array of such tests would be highly desirable if adequately validated, since liver biopsy may not be readily available in view of the large number of affected patients with hepatitis C, the risks involved in performing liver biopsies, and the problem of sampling error on biopsy that can underestimate cirrhosis in 10-30% of cases.
Standard liver biochemical tests, measures of liver function such as coagulation studies, and radiological imaging of the liver may be sufficiently sensitive to diagnose advanced cirrhosis but have not been accurate in defining evolving hepatic fibrosis and early stages of cirrhosis.44 A number of studies have been published employing a variety of indirect markers of liver fibrosis (Fibrosure and Fibrostat) including standard liver chemistries, platelet count, prothrombin index, and lipoprotein A1 concentrations. These tests have gained acceptance in Europe as alternatives to liver biopsy.45, 46 However, the utility of these tests requires further validation in prospective studies.
Direct markers of liver fibrosis are also being evaluated, including a number of serum or urinary tests, which are thought to directly reflect the deposition or removal of extracellular matrix involved in formation of fibrous tissue in the liver.44 Similar to indirect markers, these tests, such as assays for type IV collagen and hyaluronic acid, may be able to identify patients with significant fibrosis and cirrhosis. Patients with early fibrosis may also be identified. However, the limitation of both direct and indirect serological markers remains the inability to accurately define intermediate stages of hepatic fibrosis.44
Ongoing studies should help resolve this issue and put these studies in the proper context for management of patients with hepatitis C. Adequate standardization and availability of these tests will be required before they will be of value to the clinician in making decisions as to the need for liver biopsy and to begin or discontinue therapy. Future work must define an ideal algorithm for optimal use of serologic test or tests for incorporation into clinical practice. With proper validation, it is possible that such tests may be equal to or superior to liver biopsy in assessing the degree of hepatic fibrosis.
8. Screening for Hepatocellular Carcinoma
Recommendation
Patient with cirrhosis should be screened for hepatocellular carcinoma (HCC) with alpha-fetoprotein (AFP) testing and hepatic ultrasound imaging at least once yearly.
Hepatitis C is the most common predisposing factor for the development of HCC in the U.S.47, 48 Resection and local ablative treatments have long been used to treat patients with HCC. Liver transplantation is increasingly an option for patients with early HCC who are not likely to benefit from surgical resection.49 Despite these therapies, which are achieving some success, screening for HCC is controversial.50 Screening is most commonly performed with regular imaging by ultrasonography or CT-scan, and serum AFP determination. AFP testing alone has relatively low sensitivity and specificity for HCC. In a recent study of 357 patients with CHC but not HCC, 23% had an elevated serum AFP level that was independently associated with stage III/IV hepatic fibrosis, elevated level of AST, and prolonged International Normalized Ratio (INR).51
Hepatic ultrasound has greater sensitivity than AFP testing but is associated with significant rate of false positive results, particularly in the setting of regenerative nodules associated with cirrhosis. There is emerging evidence from a small number of studies that screening for HCC improves survival. For example, a prospective randomized trial conducted in China and a retrospective analysis of cirrhotic patients with chronic liver disease in Italy showed that survival was improved by surveillance for HCC probably because tumors were identified at an earlier stage.52, 53 However, the etiology of cirrhosis in these studies was heterogeneous. At present, despite the lack of firm evidence for efficacy of surveillance, it remains standard practice to screen for HCC with AFP testing and hepatic ultrasound imaging; however, the optimal interval for screening remains undetermined. A recent study indicates that biannual AFP/ annual ultrasonography is cost effective.54 There is great need for more sensitive and specific screening tests. Consideration should also be given to whether a patient undergoing screening can be expected to benefit from and tolerate treatment of HCC. Further studies are required to better define the reliability, benefits, as well as the standard of care for frequency of screening for HCC.