Clinical Guidelines for the Medical Management of Hepatitis C
G. Hepatitis C Post-exposure Management
Recommendations
At time of exposure:
- Determine the type of exposure and assess the associated risk.
- Wash wounds with soap and water; flush mucous membranes with water.
- No post-exposure prophylaxis (immune globulin or antiviral medications) is recommended.
- Counsel the exposed person regarding hepatitis C transmission risk.
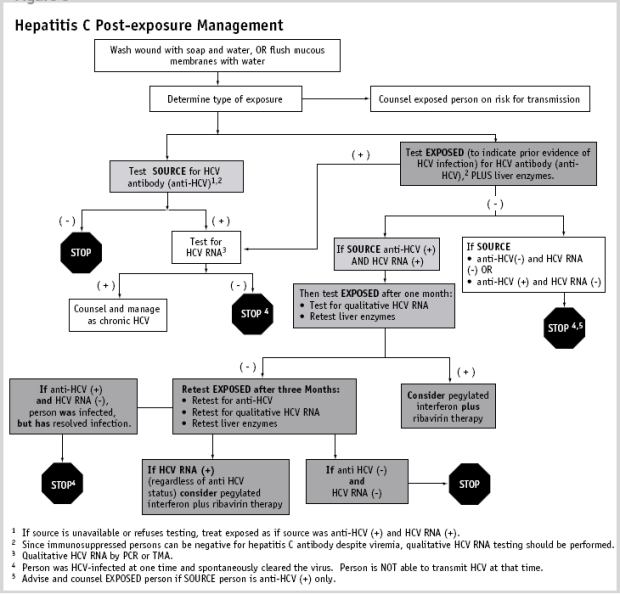
- Test source and exposed individual for hepatitis C virus antibody (figure 1) and liver enzymes for exposed individual.
- If source is not available or refuses testing, treat exposed person as if source has active hepatitis C infection.
- If source is hepatitis C virus antibody positive, or is antibody negative and is immunocompromised, test source for qualitative HCV RNA.
- If source is negative for hepatitis C antibody (and HCV RNA, if indicated), no further testing is necessary and no further action beyond initial HCV testing, is necessary for the exposed person.If source is positive for hepatitis C antibody and HCV RNA, and exposed person is negative,follow up of exposed person should be done (seeFigure 3).
The risk of transmission of HCV after a needlestick exposure from a hepatitis C-positive source is estimated at between 2-10%.114-116 This is less than the risk of hepatitis B virustransmission from a hepatitis B-positive source,but higher than the risk of HIV transmissionfrom an HIV-positive source.117 The risk oftransmission from a needlestick exposuredepends upon the concentration of HCV RNA inthe blood of the source patient and the volume ofthe inoculum. The risk of transmission of HCVfrom a single mucous membrane exposure isvery rare. Although post-exposure prophylaxiswith immune globulin was used in the past, itwas ineffective and currently no post-exposureprophylaxis is recommended. The currentrecommendations for post-exposure monitoringand management of bloodborne exposure toHCV are based on the natural history of acute hepatitis C disease and the available treatment regimens.
1. Recommendations for Post-exposure Management
The recommendations for management of bloodborne exposure to HCV are summarized below and a management algorithm is provided in Figure 3. Immediate managementafter needlestick or other bloodborne exposureincludes cleansing of wounds and mucousmembranes, determination of the natureof exposure, and post-exposure counselingas to the transmissibility of HCV and theimplications of transmission. Since post-exposure prophylaxis with immune globulin isineffective and since antiviral therapy that isdelayed until after the onset of viremia may behighly effective, no immediate post-exposuretherapy is recommended. Initially, both thesource and exposed persons need to be testedfor hepatitis C antibody. In addition, liverenzyme testing should be performed on theexposed person. If the source patient is anti-HCV negative, no further testing is needed. However, since some immunosuppressed patients are negative for hepatitis C antibody despite viremia, qualitative HCV RNA testing also may be needed at this stage. If the sourceor exposed person is positive for hepatitis Cantibody, follow-up with qualitative HCV RNAtesting is needed to determine active HCVinfection. If the source and exposed personsare anti-HCV and HCV RNA positive on initialtesting, they should be counseled and managedas in chronic hepatitis C.22
If the exposed person is anti-HCV negative initially, one month after exposure, the exposed person should be tested for qualitative HCV RNA and have liver enzymes repeated.If the exposed person is newly HCV RNApositive at this point, he/she may be offered antiviral therapy. However, because of the high rate of spontaneous clearance of infectionbetween 6-12 weeks after transmission, itis recommended to defer therapy until the results of HCV RNA testing at twelve weeksare available. Since patients with symptomsof acute hepatitis C have a higher rate ofspontaneous viral clearance, it is morestrongly recommended to defer therapy inthese patients.
If three months after the exposure, the exposed person remains anti-HCV negative, repeat testing for anti-HCV, qualitative HCV RNA, and liver enzymes. Exposed patients who arePCR positive at this point should be offeredantiviral therapy unless contraindicated.Pegylated interferon therapy should beoffered because of ease of administrationand preliminary evidence of efficacy in acutehepatitis.72,73 Ribavirin may be given as wellif there are no contraindications, although thedata on combination therapy in acute hepatitisC is even more limited. Monitoring andtreatment regimens approved for the therapyof CHC should be used.22