Medical Record Review Overview Training
September 2015
- Overview also available in Portable Document Format (PDF)
Introduction – PCG
Team Roles and Responsibilities
- Project Management: Independent Assessor (PCG)
- Oversight: Office of Quality and Patient Safety (OQPS)
- Validation: IPRO
- Medical Record Abstraction & Reporting: MedReview
Today’s Topics
- Overview of MRR purpose
- Validation Process and Quality Improvement
- Abstraction Process
- Q&A
Overview of Purpose
Office of Quality and Patient Safety
DSRIP Data Sources
Domain 1
- Implementation Plan Data
Domain 2 & 3
- Medicaid Claims and Encounters
- C & G CAHPS Survey (Medicaid insured)
- C & G CAHPS Survey (uninsured population)
- Patient Activation Measure survey
- Medical Records
- Uniform Assessment System (UAS)
- Minimum Data Set (MDS)
- Hospital Data
Domain 4
- NYS Prevention Agenda
Medical Record Review
Measures Obtained from Medical Record Data
- Screening for Clinical Depression and Follow–Up
- Controlling High Blood Pressure
- Comprehensive Diabetes Care
- Screening for all 3 tests (HbA1c test, Eye exam and Medical attention for nephropathy)
- Poor Control (>9.0%) of HbA1c
- Viral Load Suppression
- Prenatal and Post–Partum Care*
- Timeliness of Prenatal Care
- Postpartum Visits
- Frequency of Ongoing Prenatal Care (81% or more) *
- Childhood Immunization Status**
- Lead Screening in Children**
* **Measures share the same sample
Measure Relationship to Projects
| Projects | Project Descriptions | Measures with Medical Record Data |
|---|---|---|
| 3.a.i – 3.a.iv |
Integration of primary care and BH services, BH community crisis stabilization, BH medication adherence programs in community settings, withdrawal management and enhanced abstinence services within community based addiction treatment programs |
|
| 3.b.i – 3.b.ii, 3.h.i |
Evidence–based strategies for cardiovascular management in target populations, community focused primary and secondary prevention programs for cardiovascular disease, specialized medical home for chronic renal failure |
|
| 3.c.i – 3.c.ii, 3.h.i |
Evidence–based strategies for diabetes management in target populations, community focused primary and secondary prevention programs for diabetes, specialized medical home for chronic renal failure |
|
| 3.e.i | Comprehensive strategy to decrease HIV/AIDS transmission with Center of Excellence for management of HIV/AIDS |
|
| 3.f.i | Increase support programs for maternal and child health, including high risk pregnancies |
|
Cholesterol Management Measures
|
→ | Retired / removed from Measurement Year 1 |
| Replacement measures may be added to the projects in future measurement years. | ||
Determining the Population & Creating the Random Sample
| Sample Creation Steps |
|---|
|
|
|
|
|
Reconciliation of Attribution and Incorporation into Final Result
| Reconciliation Requirements |
|---|
|
|
|
|
Training & Improvement Opportunities for PPS´
Training & Improvement Opportunities for PPS´
Planning and preparation for managing medical record review in future years:
- Set up systems to coordinate requests/retrieval
- Plan staffing for abstraction
- Plan over read and coordinate the review process
- Establish data repository
- Understand validation requirements
- Produce member detail file
Measure knowledge
- Understand measure definition technicalities and codes which will facilitate work with providers and systems
- Gain an understanding of where data may be lost
- Able to abstract and work with providers throughout the year to monitor quality as improvement projects are implemented
Identification of improvement opportunities and best practices
- Tools/training needs of providers
- Member outreach needs
Validation Process and Quality Improvement – IPRO
IPRO provides a full spectrum of healthcare assessment and improvement services that foster the efficient use of resources and enhance healthcare quality to achieve better patient outcomes.
- IPRO – Improving Healthcare for the Common Good®
- ISO – 9001:2008 – CERTIFIED
- GSA Schedule Contract
- URAC – ACCREDITED INDEPENDENT REVIEW ORGANIZATION
Key Contacts
Anna Koke, IPRO
Paul Henfield, CHCA
Jeanne Alicandro, MD, IPRO
Contact Us
(516) 326–7767 ext. 589
Email address: akoke@ipro.org
IPRO´s Role
- Offer technical assistance to partners throughout the abstraction process to ensure consistency across New York
- Provide ongoing monitoring to help identify any issues early in the process
- Inform PCG, OQPS, and the PPSs of problems as they arise and offer possible solutions
- Educate PPSs regarding the measures, the record retrieval process and the accuracy of the attribution methodology used to match providers to patients
- Serve as the validator to ensure the integrity of the abstraction process
- As an NCQA Licensed Organization, act as a liaison with NCQA in instances where clarification is required
Importance of Medical Record Abstraction
Medical record abstraction can provide
- Supplemental information to augment claims information
- More specific evidence of clinical care provided than revealed by claims
For some measures claims information is incomplete
- Chart review information is used to supplement evidence of the service provided (numerators)
- Chart review information can also be used to verify population being measured (denominators/exclusions)
Screening for Clinical Depression and Follow Up Plan
Description: Percentage of Medicaid enrollees age 18 and older who were screened for clinical depression using a standardized depression screening tool, and if positive screen, received appropriate follow–up care
Purpose: Capture early identification and intervention within the context of routine preventive care visits
Eligible Population: 18 and older
- Report: ages18–64, 65 and older and total
Numerator: Members who were screened for clinical depression with a standardized tool in the measurement year and if positive, had appropriate follow up care within 30 days of the positive result.
- Requires evidence of 3 components to be compliant: screened using a tool, the result, and follow–up
Measure type: hybrid (uses either claims (G codes) or chart review information to identify the numerator)
DSRIP measurement year 1: July 1, 2014 to June 30, 2015
Relevance of the Measure
Screening for Clinical Depression and Follow Up Plan
Reflects importance of the integration of behavioral health and primary care
- Depression is commonly seen in primary care yet under–identified
- Evidence indicates that screening improves accurate identification of adults with depression in primary care settings (AHRQ)*
Consistent with DSRIP goal of achieving reduction in avoidable hospital use
- Individuals with chronic conditions, substance abuse disorders and other mental health conditions are at higher risk for developing depression
- Depression may reduce adherence to treatment plans and impair self–management of other health conditions
*AHRQ evidence synthesis Screening for Depression in Adults July 2015
Viral Load Suppression
Description: The percentage of Medicaid enrollees living with HIV/AIDS during the year prior to the measurement year who had a HIV viral load less than 200 copies/mL at last HIV viral load test during the measurement year
Eligible Population: Age 2 and older
Numerator: Number of Medicaid enrollees with a HIV viral load less than 200 copies/mL for the most recent HIV viral load test during the measurement year.
Measure type: hybrid
- Claims to identify the denominator (four methods: inpatient/ER/Outpatient visits/Pharmacy
- Medical records: to identify exclusions
- HIV negative during the measurement year or year prior
- Medical records: to determine numerator compliance
- Viral load test done and result less than 200 copies/ml
DSRIP measurement year: July 1, 2014 to June 30, 2015
Relevance of the Measure
Viral Load Suppression
New York State Prevention Agenda Focus Area 1: Prevent HIV and STDs
- Viral load suppression is associated with:
- Prolonged survival,
- Delayed disease progression
- Quality of life
- Reduced risk of HIV transmission to others
Viral load suppression is influenced by:
- Engagement and retention in care
- ART adherence, which is impacted by depression and other psychosocial barriers
New York State Prevention Agenda 2013–2017
New York State AIDS Institute HIV Clinical Resource
CDC Guidelines for the Use of Antiretroviral Agent
MMWR Diagnosis, Care, and Treatment Among Persons Living with HIV – United States, November 2014
Benefits of Validation
- Identifies specification ambiguities concurrent to the abstraction process
- Enhances the clarity of specifications
- Ongoing monitoring helps ensure abstractor consistency
- May uncover quality of care opportunities
- Provides PPSs with information for future internal quality improvement efforts
Three Components of the Validation Process
- Validation of tools used by the vendors
- Update of existing tools for the HEDIS measures
- Newly developed tools for the two non–HEDIS measures
- Chart abstraction validation– during collection (“convenience sampling”)
- Ensure that the two non–HEDIS measures are being abstracted consistently by vendors
- Uncover any specification ambiguities concurrent to the data collection process
- Chart abstraction validation – at the end of collection
- Assesses the integrity and accuracy of chart abstraction
- Determine reportability of the hybrid measures
Validation Process – At the End of Collection
- IPRO will request that the vendors provide a listing of the universe of numerator compliant cases for each measure by PPS
- IPRO will randomly sample a total of 30 cases per measure for validation
- IPRO will submit list of 30 sampled cases to the vendors
- Vendors will submit record documentation for the cases requested
- IPRO will request that the NYSDOH submit the volume of numerator compliant cases identified via claims
- IPRO will verify that the medical record documentation contains evidence of the service
- IPRO will score the accuracy by measure
- For measures that “fail” validation (i.e., the service could not be verified in the documentation submitted for a statistically significant number of cases in the sample)
- IPRO will provide the reasons for failure and ask the vendors to submit additional documentation, if available
- The vendors will be asked to undertake remedial action to correct the problems observed and IPRO will re–validate following the process outlined above
- If the second sample fails, the measure will be deemed not reportable by the PPS
- Should a measure fail, IPRO will provide recommendations for future improvement
Timeline
| Event | Timeframe |
|---|---|
| Validation of abstraction tools | September 15, 2015 |
| Validation of two–non HEDIS measures | Early in the abstraction process |
| End of Abstraction Validation | |
| Request list of numerator events | November 20, 2015 |
| Samples for validation to abstraction partner | November 30, 2015 |
| Documentation to IPRO | December 15, 2015 |
| IPRO validates record documentation | December 15 – January 15, 2016 |
| Results of the validation sent to abstraction partner | January 18, 2016 |
| Re–validation, if necessary | January 18 – January 22, 2016 |
| Final results of the validation | January 29, 2016 |
Abstraction Process
New York County Health Services Review Organization (NYCHSRO) / MedReview
URAC – Accredited Internal Independent Review Organization
URAC – Accredited Health Utilization Management
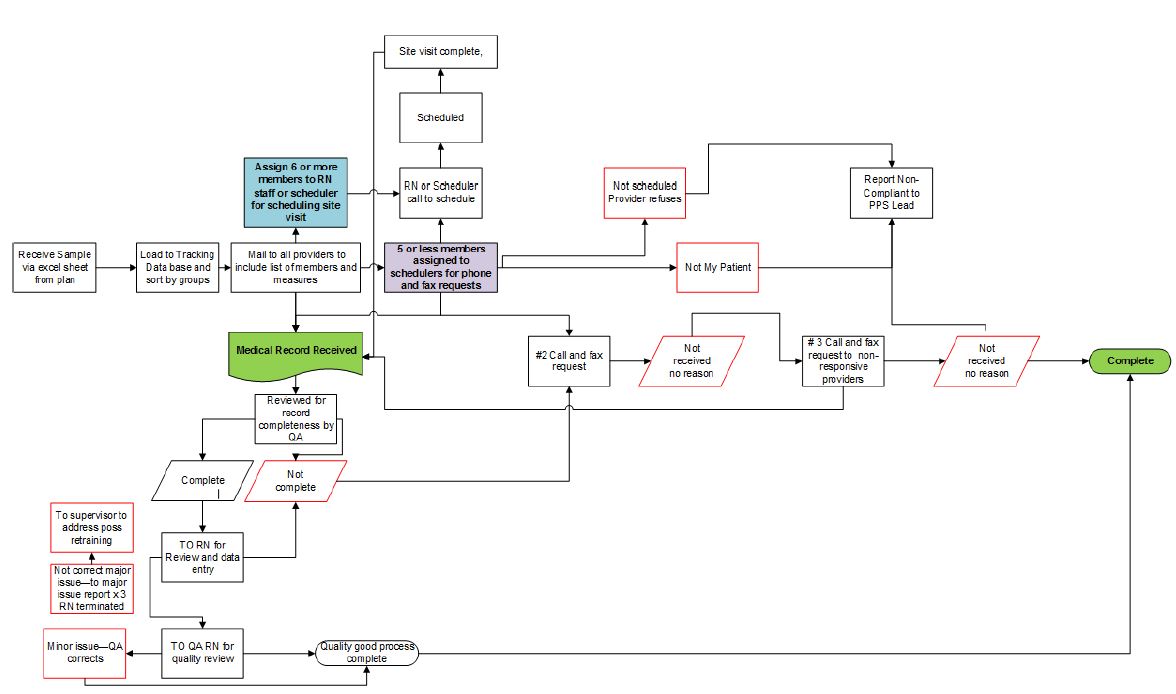
Medical Record Site Visit Collection Process
Schedule Site Visit
- Site visits will be scheduled for providers with 6 or more members at a site.
- Medical Record requests and pull sheets with member name, DOB and measure can be faxed (secure), mailed or emailed (secure) to the provider.
- Site visits are prioritized by volume and/or request.
Collect Records
- Records are collected on site by our experienced medical record collection team.
- Number of staff at the site visit is dependent on the provider.
- The staff conducting the site visit will call one day before the visit to confirm.
- All efforts should be made to assure the records are available to the staff prior to arrival.
Validate Results
- Medical record data must be validated by the collection of records by: Loading the information on a zip drive, copy and scan data, provider can mail records collected by our staff, email, secure fax and/or load medical records to a secure FTP site.
Remote Medical Record Collection
Contact Providers / Schedule Site Visits
- Remote medical record collection is conducted for sites with 5 or less members.
- Providers will be contracted via telephone within the first week of our receipt of the sample.
- Medical Record requests and pull sheets with member name, DOB and measure can be faxed (secure), mailed or emailed (secure) to the provider.
Collect Records
- Requested medical records can be returned to us by: secure fax, secure email, mail, or loaded to a secure FTP.
- All calls are tracked in a proprietary tracking system.
Provider Follow–up
- Upon completion of the first round of call requests, we will conduct the 2nd round of calls for those providers that have not complied.
- Providers will be contacted weekly until the medical record is obtained or until the end of the project, whichever occurs first.
- The 2nd round non–compliant provider list will be forwarded weekly to the project manager at PCG.
MedReview Medical Record Review Workflow
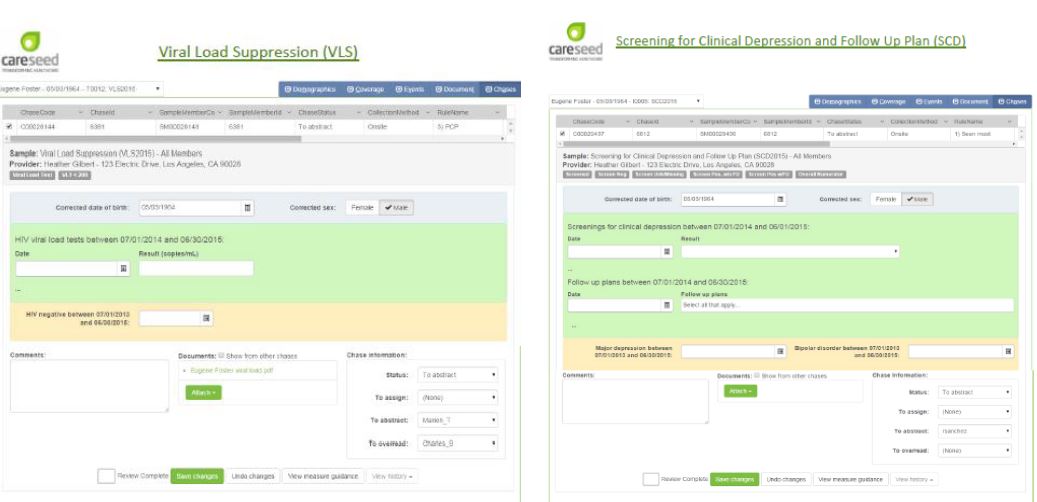
Quality Reporter Collection Template Examples
MedReview Record Collection and Review Timeline
| Event | Timeframe |
|---|---|
| Sample received | Early September |
| Initial medical request mailing/fax/schedule site visits | Mid–September |
| Medical record abstraction begins | September to November |
| Convenience sample to auditor | Mid–September |
| Follow–up mail / fax requests | Early October |
| Investigate returned mail and provider contact issues | On–going |
| Medical record review 50% complete | Mid–October |
| Medical record review 75% complete | Mid–November |
| Medical record review complete | December |
| Final medical record review validation | December |
Contact us with any and all questions
Janet Stieg RN, MS, CPHQ
- Phone: 212.897.6033
- Fax: 888.974.1099
- Janet–stieg@medreview.us
Follow Us